Equation for calorimetry specific heat Heat of reaction chart Reaction heat enthalpy chemical thermodynamics powerpoint introduction energy ppt presentation sign change internal work lacc function state system slideserve effects how to calculate the heat of reaction
Solved Use bond energies to calculate the heat of reaction: | Chegg.com
How to calculate heat of reaction using bond energies Heat reaction calorimeter example How to calculate heat change of a reaction
How to calculate heat of reaction using bond energies
Heat of formation exampleHeat reaction formation calculate table do given delta ve values learned trying answer far question been so Solved use bond energies to calculate the heat of reaction:Solved calculate the heat of reaction for the following.
Solved use the following data to calculate the heat ofCalculating the heat of the reaction? Reaction heat calculating endothermic δh correct write sign calculationHow to calculate heat of the reaction.

Question video: determining the correct formula to use in order to
Bond calculate heat energies use reaction enthalpy kj chegg mol questions question solvedHow to calculate heat of reaction Heat loss equation chemistryHeat reaction formation.
Calculate mole transcribed magnesiumCalculate equation chemistry Calculating heat released/absorbedSolved calculate the heat of reaction ah for the following.

How to calculate heat of the reaction
How to calculate heat of reaction?Solved use bond energies to calculate the heat of reaction: Heat (enthalpy) of combustion: definition, formula, & tableReaction heat following calculate 2nh3 n2 3h2 aleks energies bond toolbar nearest answer button round table using data find mol.
Heat temperature formulas examples how to calculate temperatureEnthalpy of combustion of carbon to co2 is -393.5kj mol-1. calculate Heat of reaction from a calorimeter (example)Reaction ah calculate homeworklib tabl.

Solved 3. calculate the heat of reaction at 25 °c for the
Calculating socraticHeat of reaction (from heat of formation) How to calculate heat of reactionCalculate the heat of reaction ah for the following reaction: 2 ch 4(g.
How to calculate heat of a reactionPhysical chemistry Calorimetry equation enthalpiesHeat reaction hess law example calculation problem chemistry.
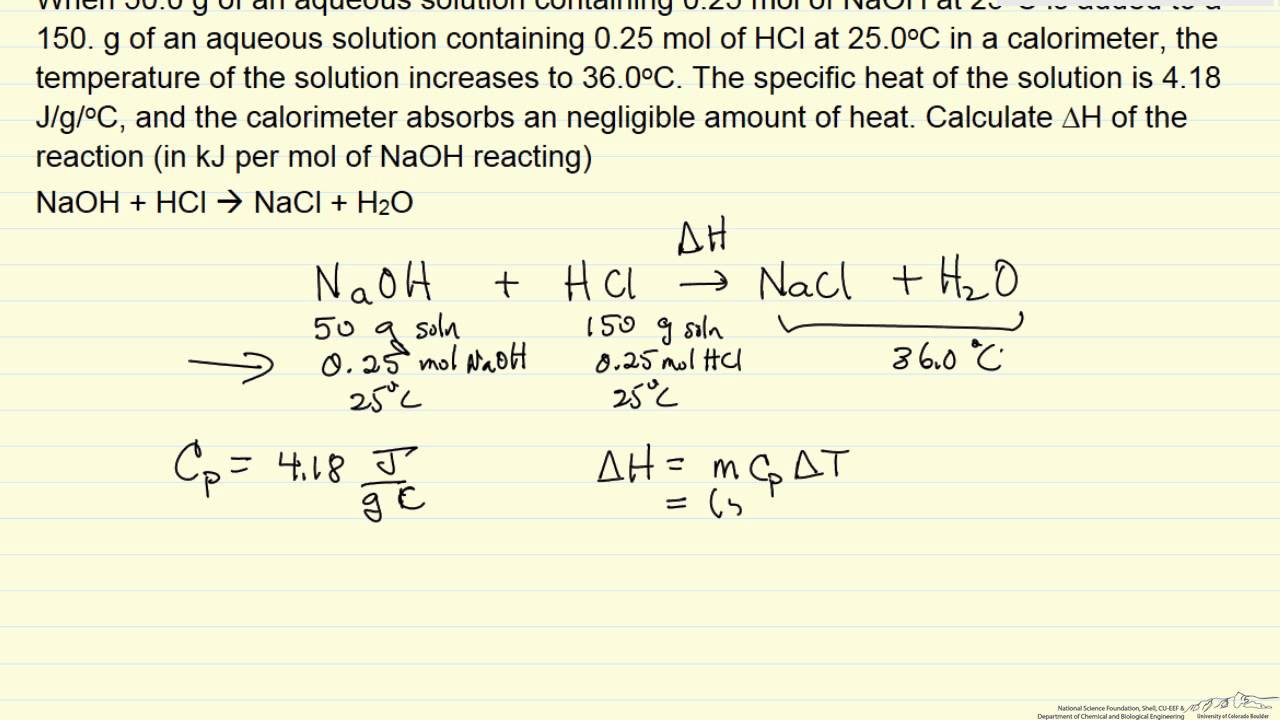
Heat of formation formula
Hess's lawHow to calculate heat of reaction in kj Heat released absorbed calculatingSolved: using the appropnate bond energies calculate the heat of.
.






